Volunteers are at the heart of our research
Experience outstanding care
Clinical research volunteers who give their precious time and energy are at the heart of all medical advancements.
That’s why, at Optimal, we work to give our volunteers the best possible experience we can.

We also offer volunteers
- Free parking
- Easy to access locations in Auckland Central, North Shore & Christchurch
- Flexible appointment times
- Pre-arranged transport, for those who need it
- Light refreshments
- Light and airy clinic rooms, and comfortable waiting areas
- Compensation for your contribution to medicine
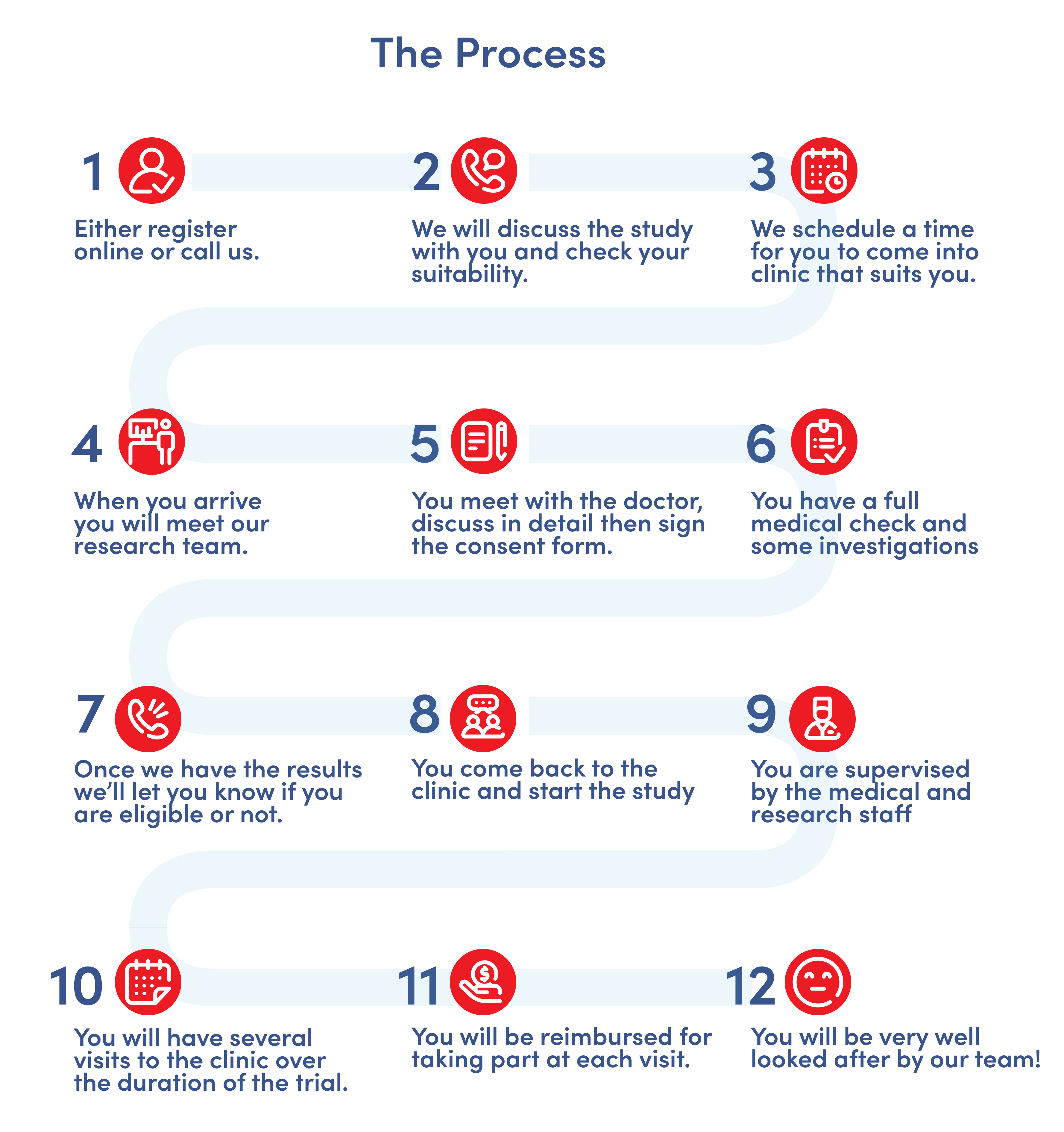
How the clinical research volunteer process works
What Clinical Trial participants are saying about their experience
"Thanks for allowing me the opportunity to take part in this study. I found the whole experience to be very pleasant and professional. Everyone was very friendly and easy to get along with, which made the time pass very quickly. I would thoroughly recommend Dr Montgomery and his team to anyone considering doing studies with them."
- Emmie C
"Friendliest group of people. Barney, and team, you're the best. Explanations and answering questions fully. Great follow-up."
- Trevor M
“Awesome experience with Optimal Clinical Trials. I always felt welcome coming in for research and everyone treated me like a friend, rather than a ‘participant’.”
- Alycia C

